Recently, Professor Jianxin Zou from the Center of Hydrogen Science at Shanghai Jiao Tong University and Professor Richard M. Laine from the University of Michigan, Ann Arbor, published the research results of constructing high-performance magnesium/lithium hybrid ion batteries based on cobalt sulfide (CoS) materials in the international well-known journal, Energy Storage Materials (IF = 17.789), titled as “Li+ assisted fast and stable Mg2+ reversible storage in cobalt sulfide cathodes for high performance magnesium/lithium hybrid-ion batteries”. Professor Jianxin Zou and Professor Richard M. Laine are the co-corresponding authors of the paper, and doctoral student, Hao Xu, is the first author of the paper.

Magnesium ion batteries (MIBs) based on magnesium anodes have attracted researchers' attention because of their high safety and low cost. However, the further development of MIBs is limited by their low actual energy density, poor reversibility and short cycle life. Magnesium/lithium hybrid ion batteries (MLHBs) have the advantages of MIBs because they can combine Li+ with high reaction activity and magnesium anodes with high capacity and low tendency to form dendrites. However, compared with MIBs, MLHBs show more excellent electrochemical performance, such as higher charge/discharge specific capacities and longer cycle life. Cathode materials are generally divided into intercalation- and conversion-type materials. Intercalation-type cathodes often show low actual specific capacities, while conversion-type cathodes have high theoretical/actual specific capacities, which has attracted more attentions. Among them, cobalt sulfide (CoS) has high theoretical specific capacity (589 mAh g-1), low cost and simple synthesis process. It is an ideal cathode material for MLHBs.
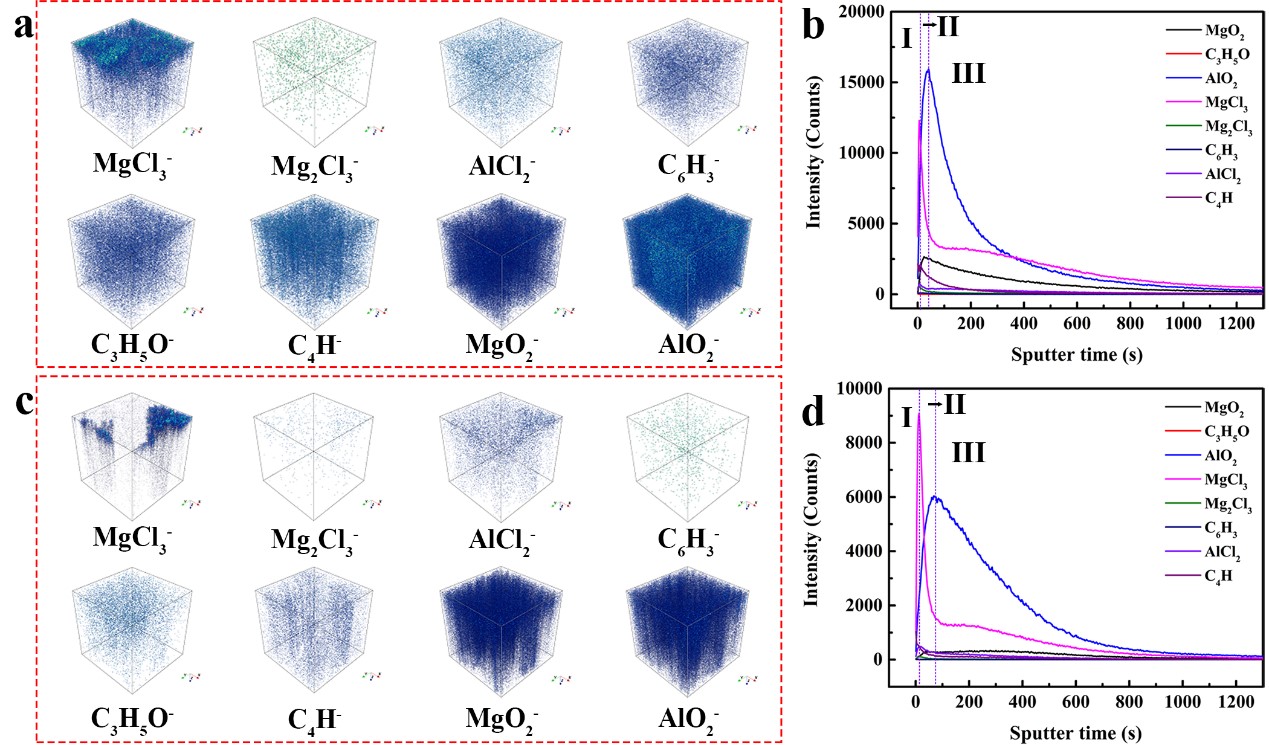
Fig. 1. TOF-SIMS 3D rendering models of MgCl3-, Mg2Cl3-, AlCl2-, C6H3-, C3H5O-, C4H-, MgO2-, AlO2- and the intensity evolution of various species as a function of the sputtering time for the deposits on the cycled Mg electrodes derived from (a, b) APC-0.8LiCl and (c, d) APC electrolytes.
In this study, Professor Jianxin Zou's team synthesized a series of electrolytes based on all phenyl complex (APC) electrolyte, including APC-0.2 LiCl, APC-0.2 LiF, APC-0.2 LiTFSI and APC-0.2 NaCl. The test results of symmetrical Mg battery show that APC-0.2 LiCl electrolyte is more beneficial for the stripping/plating of Mg2+, and the Mg content of magnesium surface deposit after battery cycling in this electrolyte is the highest, which confirms the synergistic effect of Li+ and Cl- in APC electrolyte. Then the concentration of LiCl in APC electrolyte was further increased. The results show that APC-0.8 LiCl electrolyte prepared by adding 0.8 M LiCl has excellent electrochemical performance. TOF-SIMS test was carried out on the magnesium surface deposits obtained from APC and APC-0.8 LiCl electrolyte. The results show that LiCl can improve the uniformity of effective deposits on the surface of magnesium foil.

Fig. 2. (a-g) Electrochemical evaluation of CoS cathodes using APC-0.8 LiCl electrolyte. (h) Electrochemical performance comparison of CoS with other typical cathode materials for MLHBs reported in the literatures
MLHBs with CoS cathode, APC-0.8 LiCl electrolyte and magnesium anodes were assembled. The cyclic voltammetry analyses of MLHBs show that there is an obvious polarization at 1.8 V. In the process of galvanostatic (100 mA g-1) charge and discharge, MLHBs experience an obvious activation process (voltage window: 0.01-1.8 V). After 26 cycles, the specific capacities of charge and discharge tend to be stable. After 80 cycles, the specific capacities remain at 538 mAh g-1. Moreover, after the complete activation process, the voltage window is expanded to 0.01-2 V. The cyclic voltammetry results show that the polarization of the cathode will not increase. On the contrary, the MLHBs show good rate performance. At 1000 mA g-1, the MLHBs maintain a very high specific capacity of 320 mAh g-1 after 1000 cycles. The MLHBs have high specific capacities and long cycle life, which shows a wide application prospect in the field of energy storage.

Fig. 3. (a, b) The voltage-time curve of CoS cathode in various states (I-VII) during 1st cycle and corresponding ex-situ XRD patterns. The XPS spectra of CoS cathodes in different states. (f) TEM image, (g) SAED pattern and (h, i) HRTEM images of CoS cathode in fully charged state (cycle 50). (j, k) The sXAS spectra of CoS cathodes in different states. (l-m) Kinetic analyses of electrochemical behavior for MLHBs.
The density functional theory (DFT) calculation results show that Li+ has a lower energy change than Mg2+ when embedded in CoS, which proves that the embedding of Li+ mainly occurs in the initial discharge process during MLHB working. Simultaneously, the embedding of Li+/Mg2+ is conducive to improving the density of states at the Fermi level of CoS, thus enhancing the conductivity of CoS cathode. The in-situ XRD, XPS, TEM, SEM, EDX and sXAS results show that Li+ is easier to be embedded into CoS lattice than Mg2+, as well as playing the role of shielding charge. With the further embedding of Li+, CoS lattice is distorted and Co9S8 phase is formed. In the initial stage of charge and discharge (such as the 1st cycle), the specific capacities of the MLHBs mainly come from the reaction between Li+ and CoS cathode material (Fig. 4a, equation 1). After full activation (such as the 50th cycle), the specific capacities of the MLHBs mainly come from the reaction between Mg2+ and Co9S8 (Fig. 4b, equation 2). Mg2+ and Li+ participate in the cathode reactions in the whole charge and discharge process, thus contributing to the specific capacities of the MLHBs. In addition, the crystal structure of CoS (P 63/mmc) is very different from that of the target product MgS (Fm-3m), while Co9S8 (Fm-3m) has the same crystal structure with CoS and MgS. Therefore, Co9S8 has higher reaction reversibility than CoS when reacting with Mg2+ to produce Co and MgS, which is conducive to the stable and long cycle of MLHBs. Furthermore, Co9S8 was directly assembled into MLHBs. It was found that the activation time of the MLHBs decreased sharply when compared with that using CoS, confirming the above conclusion. The pseudo-capacitance test results show that the specific capacitance contribution of MLHBs is large, conducive to improving the rate performance of the MLHBs. This work provides a new idea for the research of multivalent ion batteries (such as Mg2+, Ca2+, Al3+ and Zn2+), emphasizing the auxiliary effect of secondary ions (such as H+, NH4+, Na+). In addition, the reasonable preparation of conversion-type cathode materials with crystal structure similar to the target product is an effective strategy for the assembly of high-performance MIBs and MLHBs.
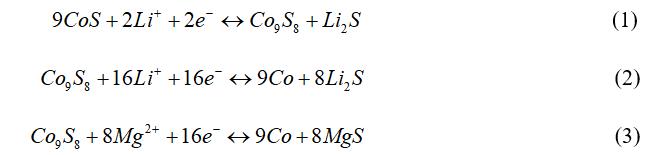
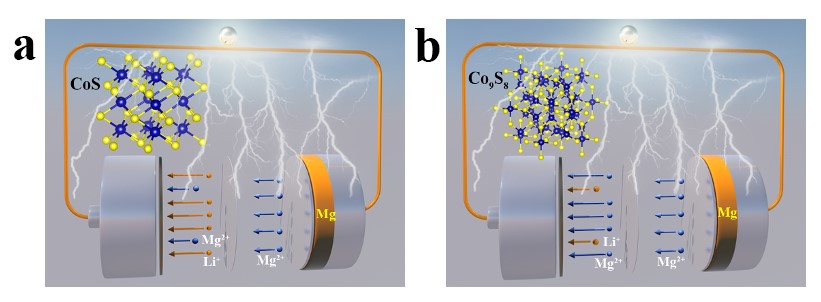
Fig. 4. Schematic diagrams of MLHB working during (a) initial cycles and (b) after activation.
This work has been supported by the "Zhiyuan Honor Program" for doctoral students of Shanghai Jiao Tong University, and supported by the Hydrogen Science Center of Shanghai Jiao Tong University, the National Natural Science Foundation of China, Shanghai Science and Technology Commission, Shanghai Education Commission.
Paper link: https://doi.org/10.1016/j.ensm.2022.01.041
Author: Hao Xu
Source: Center of Hydrogen Science, Shanghai Jiao Tong University
