As parts of our innate immunity, polymorphonuclear neutrophils release their chromatin into extracellular environment to fight against invading pathogens. This chromatin-derived antimicrobial materials, called neutrophil extracellular traps (NETs), can efficiently ensnare bacteria and alert immune cells for phagocytosis and secretion of interleukins. However, for immunity-compromised patients, the excessive formation or slow degradation of NETs in vivo causes unexpected pathological effects, such as impaired antimicrobial activity, increased tissue toxicity and many immune disorder diseases.
Researchers in the field tend to isolate NETs from patients, and then disclose the pathological roles of NETs from their abnormal compositions, properties and functions. However, NETs are highly dynamic and complexed structures composed of DNA, dozens of antimicrobial peptides and enzymes, making a real challenge to dissect the pathological implication of NETs. The inaccessibility to disclose NET-related pathology also comes from the complexed tissue environment, such as responses of immune cells, release of cytokines from tissue cells, and coordination of complement systems.
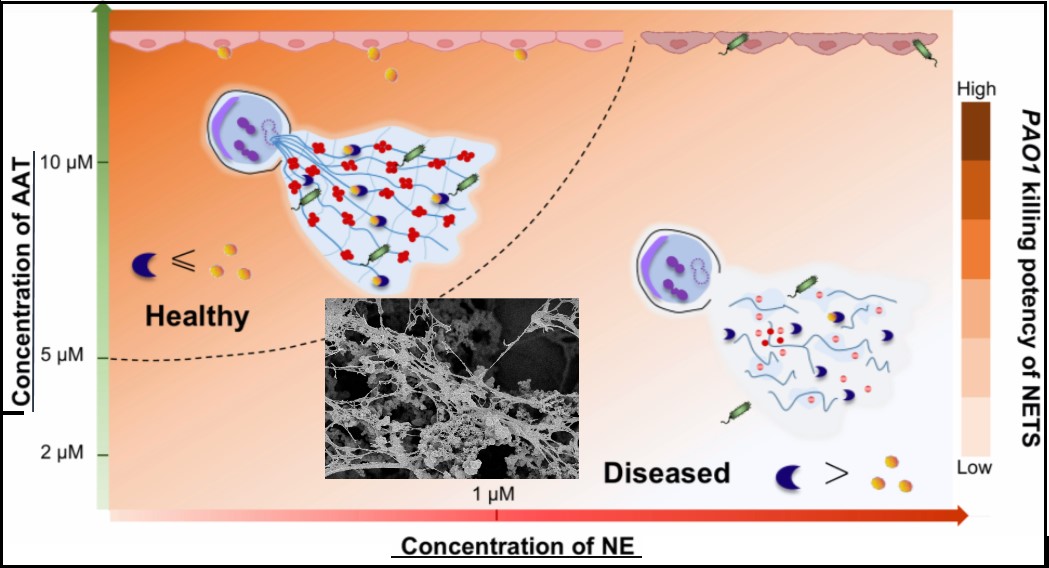
To decipher the composition-dependent physiological functions of NETs, Dr. Yang Song and his lab members from School of MSE, SJTU, have now developed a bottom-up approach to synthesize recombinant NETs, by a step-wise control over the composition and supramolecular structure of DNA, histone and other important antimicrobial enzymes. The synthesized NETs-mimetic material can efficiently trap bacteria, stimulate immune cell responses and even phagocytosis. By further comparing the antimicrobial activity of synthetic NETs with endogenous NETs, these researchers have developed a biomimetic material-synthesis approach to disclose the causal relationship between NETs structure and their antimicrobial functions.
"The synthetic NETs are not just antimicrobial materials, but they could be a powerful tool to dissect antimicrobial potency of NETs", said Dr. Yang Song, the primary investigator of this research program in MSE, SJTU. Since initial collaboration with Prof. S. Takayama in Georgia Tech three years ago, Dr. Y. Song’s group have examined the antimicrobial behaviors of synthetic NETs in an simulated lung environment of alpha-1 antitrypsin deficiency (AATD) patients. They have confirmed that the hyperactivity of neutrophil elastase or unusual low levels of alpha-1 antitrypsin could impair the antimicrobial activity of NETs, due to the enzyme-mediated degradation of histone and other antimicrobial peptides.
"This biomimetic material-synthesis approach provides a reproducible and quantitative platform to model the NETs-related immune diseases", suggested by Dr. Y. Song. He expects that this synthetic material could assist the development of new therapies for treatment of NETs-related autoimmune diseases in the near future. The current work, entitled “Synthetic neutrophil extracellular traps dissect bactericidal contribution of NETs under regulation of α1-antitrypsin” is recently published in Science Advance, 2023 Apr; 9(17): eadf2445. Dr. Ting Yang and Jinlong Yu are co-first authors of this paper; Dr. Yang Song (SJTU) and Prof. S. Takayama (Georgia Tech) are corresponding authors.
See paper details in the following link,
